Heart disease is one of the leading causes of death in North America, with over 26 million cases in Canada and the United States combined. A new research finding provides a clue for therapeutic development.
A research team at University of British Columbia solved an intricate interaction between a key protein in muscle contraction and its regulatory molecule, which holds a promise for disease treatment.
“[This study] improves our basic understanding of disease…and in general of a protein that is a major therapeutic target in heart failure and skeletal muscle disorders,” says Filip Van Petegem, a principle investigator who headed the study.
Flux of calcium ions regulates a variety of vital bodily functions, from contraction of the heart to memory formation. Proteins called Ryanodine Receptors (RyRs) control this flux and are, conversely, regulated by a handful of molecules.
As a tap controls the flow of water, RyR allows the flow of calcium ions in spurts, which signals muscle contraction and relaxation. When its regulation fails and RyR becomes “leaky,” calcium trickles out constantly and cannot create spurts.
Leaky RyRs are associated with an array of diseases, such as muscular dystrophy, heart failure, diabetes, and Alzheimer’s. The exact mechanisms of these disorders, however, have remained elusive due to the complexity and the lack of detailed structural understanding of how the protein and its modulatory partners come into contact.
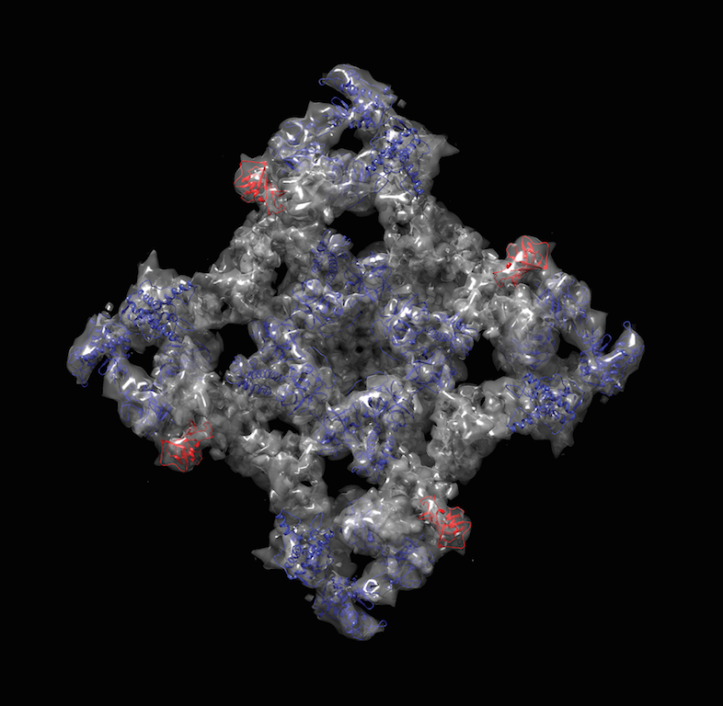
The new study reveals a high-resolution structure of a section of RyR and identifies its location within the whole protein. This segment interacts with one of the major modulators of RyRs, the research team reports.

The modulator is known to shut down RyR, equivalent to tightening the tap and stopping the leak. Scientists postulate that the modulator falling off of RyR causes diseases associated with leaky RyRs. This means that studying how the modulator interacts with RyR and coming up with molecules that can bind better could be a promising therapeutic route.
For example, compounds called rycals, developed by other research groups, are currently undergoing clinical trials. These compounds are thought to stabilize the modulator-RyR interaction and could become treatment options for hypertension, chest pain, and other heart diseases.
However, until now, the major obstacle in developing the stabilizers had been the lack of knowledge of the exact nature and location of the interaction.
“The current high-resolution structures might help the development of novel stabilizers of [modulator]-RyR interactions, since we’ve been able to pinpoint a point-to-point contact between the RyR and [the modulator],” Van Petegem says.
The researchers used a technique called X-ray crystallography to obtain detailed structural elements of the proteins, down to their individual amino acids. The method entails coaxing a solution of pure proteins into uniform crystals and exposing them to powerful X-ray beams generated by a synchrotron.
“[The study] is a useful structural template to understand [RyR] regulation and will help structure-based drug design to stabilize [modulator]-binding and treat heart failure,” says Zhiguang (Michael) Yuchi, who is the lead author of the study and postdoctoral fellow at the Van Petegem Laboratory.
Van Petegem’s team has been pursuing high-resolution structures of individual RyR segments, then piecing them back together into the lower-resolution, overall structure of RyR created by electron microscopy. Using this “divide and conquer” approach, or docking, the team has pieced together close to 20% of the entire protein to date.
Although the study provides new insights and advancement to the field, the research is far from complete. “[The] next important question is to figure out how the many small molecules are able to bind and regulate RyRs,” Van Petegem says. “This includes molecules of basic importance [that naturally occur in the body], but also of pharmacological importance.”
For example, some anesthetics and muscle relaxants are known to cause malignant hyperthermia in people with certain genetic variants of RyR. The where and how of these interactions remain open to further explorations.
“For sure X-ray crystallography will be very important to answer these questions,” Van Petegem says.
The study was led by researchers at the Life Sciences Institute in University of British Columbia, Vancouver, in collaboration with University of Minnesota and Brigham and Women’s Hospital, Boston. The report is published in the latest online issue of Nature Communications and is open access (it’s free).